ACT003-
Ubiquitin Ligase "Z"
~A Novel Molecular Targeted Therapy to Eradicate Cancer Stem Cells~
Targeting the "Source of Cancer Recurrence and Metastasis"
One of the biggest challenges in cancer treatment is "cancer recurrence and metastasis."
Recently, special cells called cancer stem cells, which possess self-renewal ability and high drug resistance, have attracted attention as the cause of these phenomena.
We are working on developing treatments aimed at fundamentally eliminating these cancer stem cells.
Discovery of Cancer Stem Cell-Specific Ubiquitin Ligase "Z"
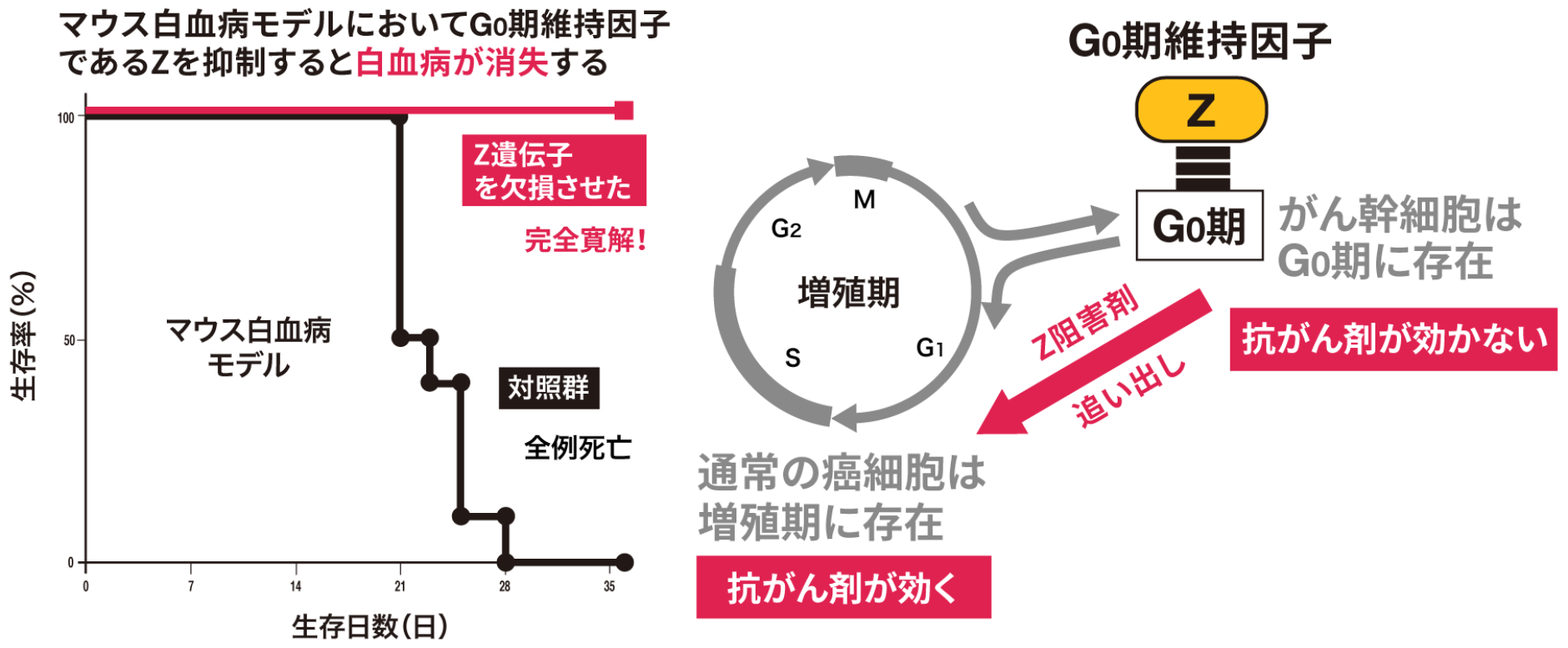
Our research team was the first in the world to discover Ubiquitin Ligase "Z (tentative name)," which is specifically expressed in cancer stem cells.
When the Z gene was artificially deleted within cancer cells, the cancer stem cells could not survive and were eliminated, leading to the shrinkage and elimination of the entire cancer. We demonstrated this.
This achievement presents a new possibility that "targeting cancer stem cells leads to cancer eradication."
Innovative Inhibitor Targeting the Ubiquitin System
We are currently searching for small molecule compounds (inhibitors) that inhibit the function of Ubiquitin Ligase Z.
Since Z is hardly expressed in normal cells and is an enzyme specific to cancer stem cells, it is expected to be a treatment method with few side effects.
Future Prospects
We will optimize inhibitor candidates and evaluate their efficacy and safety in cell and animal experiments.
We aim for it to become a pillar of next-generation cancer treatment as a "pan-cancer compatible molecular targeted drug" that can be used regardless of cancer type.
Frequently Asked Questions (FAQ)
Q1. What kind of protein is Ubiquitin Ligase Z?
A. Ubiquitin Ligase Z is a protein specifically expressed in cancer stem cells and is involved in ubiquitination, which controls protein degradation within cells.
Inhibiting Z makes cancer stem cell survival impossible, leading to the shrinkage and elimination of the entire cancer.
Q2. Is this inhibitor effective against all cancers?
A. Current research confirms that Z is commonly expressed in multiple types of cancer stem cells, suggesting potential application regardless of cancer type.
However, further research and verification are necessary for clinical application.
Q3. Are there concerns about safety or side effects?
A. Since Z is hardly expressed in normal cells, it is highly target-specific, and the risk of side effects is considered low.
We plan to thoroughly conduct safety evaluations in future preclinical trials.